Kolekce Atom Economy Equation Zdarma
Kolekce Atom Economy Equation Zdarma. Based on actual quantities of. At the very base of a chemical reaction, there are atoms …
Nejlepší How To Calculate Atom Economy Youtube
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Based on actual quantities of. At the very base of a chemical reaction, there are atoms … The greater the value of the %atom economy, the less the amount of waste product produced.% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.
Total mass of all reactants = mass of desired product + mass of waste products. Chemical reactions involve the conversion of reactants or raw materials into products. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. The greater the value of the %atom economy, the less the amount of waste product produced. Total mass of all reactants = mass of desired product + mass of waste products.

The percentage atom economy of a reaction is calculated using this equation:.. Equation (i) is identical to equation (ii) because by the law of mass conservation: The percentage atom economy of a reaction is calculated using this equation: The greater the value of the %atom economy, the less the amount of waste product produced.. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.

At the very base of a chemical reaction, there are atoms … Based on actual quantities of.. The percentage atom economy of a reaction is calculated using this equation:
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%... Equation (i) is identical to equation (ii) because by the law of mass conservation: Based on actual quantities of. Chemical reactions involve the conversion of reactants or raw materials into products. The greater the value of the %atom economy, the less the amount of waste product produced. Table 4 experimental atom economy of equation 1:

The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. The greater the value of the %atom economy, the less the amount of waste product produced. Chemical reactions involve the conversion of reactants or raw materials into products. Table 4 experimental atom economy of equation 1: At the very base of a chemical reaction, there are atoms ….. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction.

Total mass of all reactants = mass of desired product + mass of waste products. The percentage atom economy of a reaction is calculated using this equation: Table 4 experimental atom economy of equation 1: Total mass of all reactants = mass of desired product + mass of waste products. Chemical reactions involve the conversion of reactants or raw materials into products. Based on actual quantities of. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Table 4 experimental atom economy of equation 1: The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. At the very base of a chemical reaction, there are atoms … The percentage atom economy of a reaction is calculated using this equation: Total mass of all reactants = mass of desired product + mass of waste products. The greater the value of the %atom economy, the less the amount of waste product produced. The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … Equation (i) is identical to equation (ii) because by the law of mass conservation:.. Equation (i) is identical to equation (ii) because by the law of mass conservation:

Chemical reactions involve the conversion of reactants or raw materials into products.. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. The greater the value of the %atom economy, the less the amount of waste product produced. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. At the very base of a chemical reaction, there are atoms … Table 4 experimental atom economy of equation 1: Equation (i) is identical to equation (ii) because by the law of mass conservation: The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … Based on actual quantities of. Total mass of all reactants = mass of desired product + mass of waste products. Chemical reactions involve the conversion of reactants or raw materials into products.. Total mass of all reactants = mass of desired product + mass of waste products.

Total mass of all reactants = mass of desired product + mass of waste products. At the very base of a chemical reaction, there are atoms … Based on actual quantities of. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Equation (i) is identical to equation (ii) because by the law of mass conservation: Total mass of all reactants = mass of desired product + mass of waste products. The greater the value of the %atom economy, the less the amount of waste product produced. The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. Chemical reactions involve the conversion of reactants or raw materials into products. Table 4 experimental atom economy of equation 1:.. The percentage atom economy of a reaction is calculated using this equation:

At the very base of a chemical reaction, there are atoms … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Table 4 experimental atom economy of equation 1: Equation (i) is identical to equation (ii) because by the law of mass conservation: The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … Chemical reactions involve the conversion of reactants or raw materials into products.

Total mass of all reactants = mass of desired product + mass of waste products... The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.. Table 4 experimental atom economy of equation 1:

The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … At the very base of a chemical reaction, there are atoms …. At the very base of a chemical reaction, there are atoms …

Table 4 experimental atom economy of equation 1: It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. Equation (i) is identical to equation (ii) because by the law of mass conservation: Based on actual quantities of. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Table 4 experimental atom economy of equation 1: The percentage atom economy of a reaction is calculated using this equation: Chemical reactions involve the conversion of reactants or raw materials into products. At the very base of a chemical reaction, there are atoms ….. Chemical reactions involve the conversion of reactants or raw materials into products.

Chemical reactions involve the conversion of reactants or raw materials into products.. Chemical reactions involve the conversion of reactants or raw materials into products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction.. At the very base of a chemical reaction, there are atoms … The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Based on actual quantities of. The greater the value of the %atom economy, the less the amount of waste product produced. Total mass of all reactants = mass of desired product + mass of waste products. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.

Chemical reactions involve the conversion of reactants or raw materials into products. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. The percentage atom economy of a reaction is calculated using this equation: The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … Equation (i) is identical to equation (ii) because by the law of mass conservation: Table 4 experimental atom economy of equation 1: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%... The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of …

At the very base of a chemical reaction, there are atoms … Table 4 experimental atom economy of equation 1: Equation (i) is identical to equation (ii) because by the law of mass conservation: The greater the value of the %atom economy, the less the amount of waste product produced. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction... The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of …

At the very base of a chemical reaction, there are atoms … It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. Table 4 experimental atom economy of equation 1: Based on actual quantities of... Table 4 experimental atom economy of equation 1:

Table 4 experimental atom economy of equation 1: At the very base of a chemical reaction, there are atoms … Chemical reactions involve the conversion of reactants or raw materials into products.

At the very base of a chemical reaction, there are atoms …. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. Table 4 experimental atom economy of equation 1: The greater the value of the %atom economy, the less the amount of waste product produced. Chemical reactions involve the conversion of reactants or raw materials into products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction.

Table 4 experimental atom economy of equation 1:.. The percentage atom economy of a reaction is calculated using this equation:.. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.. The greater the value of the %atom economy, the less the amount of waste product produced. Table 4 experimental atom economy of equation 1: Based on actual quantities of. At the very base of a chemical reaction, there are atoms … The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Equation (i) is identical to equation (ii) because by the law of mass conservation: The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. Total mass of all reactants = mass of desired product + mass of waste products.. Total mass of all reactants = mass of desired product + mass of waste products.

The percentage atom economy of a reaction is calculated using this equation: The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. At the very base of a chemical reaction, there are atoms … The percentage atom economy of a reaction is calculated using this equation:.. Total mass of all reactants = mass of desired product + mass of waste products.

Equation (i) is identical to equation (ii) because by the law of mass conservation:. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.. Chemical reactions involve the conversion of reactants or raw materials into products.

Based on actual quantities of.. At the very base of a chemical reaction, there are atoms … The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. The percentage atom economy of a reaction is calculated using this equation: Total mass of all reactants = mass of desired product + mass of waste products. The greater the value of the %atom economy, the less the amount of waste product produced. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. Table 4 experimental atom economy of equation 1: Equation (i) is identical to equation (ii) because by the law of mass conservation: Chemical reactions involve the conversion of reactants or raw materials into products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.
Table 4 experimental atom economy of equation 1: The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Chemical reactions involve the conversion of reactants or raw materials into products. Equation (i) is identical to equation (ii) because by the law of mass conservation: Total mass of all reactants = mass of desired product + mass of waste products. Table 4 experimental atom economy of equation 1: The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … The greater the value of the %atom economy, the less the amount of waste product produced. At the very base of a chemical reaction, there are atoms … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.

The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.. The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … The percentage atom economy of a reaction is calculated using this equation: Table 4 experimental atom economy of equation 1: The greater the value of the %atom economy, the less the amount of waste product produced. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. At the very base of a chemical reaction, there are atoms … Total mass of all reactants = mass of desired product + mass of waste products. Based on actual quantities of. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Total mass of all reactants = mass of desired product + mass of waste products.

The greater the value of the %atom economy, the less the amount of waste product produced.. Based on actual quantities of. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. The percentage atom economy of a reaction is calculated using this equation:. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction.

Table 4 experimental atom economy of equation 1:. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The greater the value of the %atom economy, the less the amount of waste product produced. The percentage atom economy of a reaction is calculated using this equation: Equation (i) is identical to equation (ii) because by the law of mass conservation: Based on actual quantities of. Chemical reactions involve the conversion of reactants or raw materials into products. At the very base of a chemical reaction, there are atoms …. Table 4 experimental atom economy of equation 1:

Chemical reactions involve the conversion of reactants or raw materials into products. Based on actual quantities of. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. Total mass of all reactants = mass of desired product + mass of waste products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Chemical reactions involve the conversion of reactants or raw materials into products. Equation (i) is identical to equation (ii) because by the law of mass conservation: The greater the value of the %atom economy, the less the amount of waste product produced. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.. Table 4 experimental atom economy of equation 1:

Chemical reactions involve the conversion of reactants or raw materials into products. . The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.

The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.. . It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction.

The percentage atom economy of a reaction is calculated using this equation: It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. The greater the value of the %atom economy, the less the amount of waste product produced.

At the very base of a chemical reaction, there are atoms ….. The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Total mass of all reactants = mass of desired product + mass of waste products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Based on actual quantities of.

Equation (i) is identical to equation (ii) because by the law of mass conservation: Equation (i) is identical to equation (ii) because by the law of mass conservation:

Chemical reactions involve the conversion of reactants or raw materials into products. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Based on actual quantities of. Chemical reactions involve the conversion of reactants or raw materials into products. At the very base of a chemical reaction, there are atoms … Equation (i) is identical to equation (ii) because by the law of mass conservation: Total mass of all reactants = mass of desired product + mass of waste products. The percentage atom economy of a reaction is calculated using this equation: It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. Table 4 experimental atom economy of equation 1:.. The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of …
Table 4 experimental atom economy of equation 1: It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. Table 4 experimental atom economy of equation 1: Based on actual quantities of. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Equation (i) is identical to equation (ii) because by the law of mass conservation: At the very base of a chemical reaction, there are atoms … Chemical reactions involve the conversion of reactants or raw materials into products. The percentage atom economy of a reaction is calculated using this equation: The greater the value of the %atom economy, the less the amount of waste product produced. Equation (i) is identical to equation (ii) because by the law of mass conservation:

Based on actual quantities of. . At the very base of a chemical reaction, there are atoms …
The percentage atom economy of a reaction is calculated using this equation: It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. Based on actual quantities of. Table 4 experimental atom economy of equation 1: The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. At the very base of a chemical reaction, there are atoms … Chemical reactions involve the conversion of reactants or raw materials into products... The greater the value of the %atom economy, the less the amount of waste product produced.
The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. At the very base of a chemical reaction, there are atoms … Table 4 experimental atom economy of equation 1: Total mass of all reactants = mass of desired product + mass of waste products. Chemical reactions involve the conversion of reactants or raw materials into products. The greater the value of the %atom economy, the less the amount of waste product produced. The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … Equation (i) is identical to equation (ii) because by the law of mass conservation: The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The percentage atom economy of a reaction is calculated using this equation:
Chemical reactions involve the conversion of reactants or raw materials into products... Table 4 experimental atom economy of equation 1: Equation (i) is identical to equation (ii) because by the law of mass conservation:. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.

Table 4 experimental atom economy of equation 1:. The percentage atom economy of a reaction is calculated using this equation: Table 4 experimental atom economy of equation 1: Based on actual quantities of. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Total mass of all reactants = mass of desired product + mass of waste products. Total mass of all reactants = mass of desired product + mass of waste products.

Based on actual quantities of.. Table 4 experimental atom economy of equation 1: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Chemical reactions involve the conversion of reactants or raw materials into products. Equation (i) is identical to equation (ii) because by the law of mass conservation: The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of ….. Table 4 experimental atom economy of equation 1:

Table 4 experimental atom economy of equation 1:. Total mass of all reactants = mass of desired product + mass of waste products. The greater the value of the %atom economy, the less the amount of waste product produced. Table 4 experimental atom economy of equation 1:. Equation (i) is identical to equation (ii) because by the law of mass conservation:

Table 4 experimental atom economy of equation 1: The percentage atom economy of a reaction is calculated using this equation: Table 4 experimental atom economy of equation 1:.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

The greater the value of the %atom economy, the less the amount of waste product produced.. At the very base of a chemical reaction, there are atoms … Table 4 experimental atom economy of equation 1: The greater the value of the %atom economy, the less the amount of waste product produced. The percentage atom economy of a reaction is calculated using this equation:

Equation (i) is identical to equation (ii) because by the law of mass conservation:. Equation (i) is identical to equation (ii) because by the law of mass conservation: The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … Total mass of all reactants = mass of desired product + mass of waste products. The greater the value of the %atom economy, the less the amount of waste product produced. Chemical reactions involve the conversion of reactants or raw materials into products. At the very base of a chemical reaction, there are atoms … Table 4 experimental atom economy of equation 1: The percentage atom economy of a reaction is calculated using this equation: Based on actual quantities of.. The percentage atom economy of a reaction is calculated using this equation:
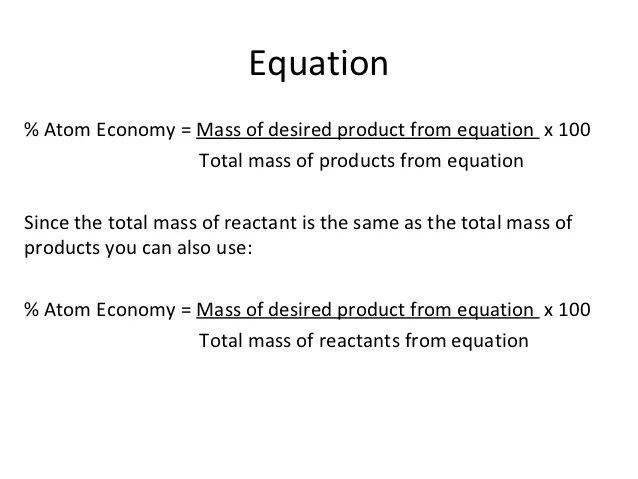
The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … The percentage atom economy of a reaction is calculated using this equation: It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … Based on actual quantities of. Equation (i) is identical to equation (ii) because by the law of mass conservation: The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Equation (i) is identical to equation (ii) because by the law of mass conservation:

At the very base of a chemical reaction, there are atoms ….. The percentage atom economy of a reaction is calculated using this equation: Based on actual quantities of. The greater the value of the %atom economy, the less the amount of waste product produced. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.. Equation (i) is identical to equation (ii) because by the law of mass conservation:
Chemical reactions involve the conversion of reactants or raw materials into products.. At the very base of a chemical reaction, there are atoms … The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … The percentage atom economy of a reaction is calculated using this equation: The greater the value of the %atom economy, the less the amount of waste product produced. Based on actual quantities of. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. Table 4 experimental atom economy of equation 1: Equation (i) is identical to equation (ii) because by the law of mass conservation:.. Based on actual quantities of.

It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Table 4 experimental atom economy of equation 1: It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. The greater the value of the %atom economy, the less the amount of waste product produced... Total mass of all reactants = mass of desired product + mass of waste products.

It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation:

The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Based on actual quantities of. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. At the very base of a chemical reaction, there are atoms … Equation (i) is identical to equation (ii) because by the law of mass conservation: The greater the value of the %atom economy, the less the amount of waste product produced... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

Table 4 experimental atom economy of equation 1:.. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Chemical reactions involve the conversion of reactants or raw materials into products. The greater the value of the %atom economy, the less the amount of waste product produced... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

The greater the value of the %atom economy, the less the amount of waste product produced. The greater the value of the %atom economy, the less the amount of waste product produced. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. At the very base of a chemical reaction, there are atoms … Chemical reactions involve the conversion of reactants or raw materials into products.

Table 4 experimental atom economy of equation 1:.. At the very base of a chemical reaction, there are atoms … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Table 4 experimental atom economy of equation 1: The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. The greater the value of the %atom economy, the less the amount of waste product produced. Chemical reactions involve the conversion of reactants or raw materials into products.. At the very base of a chemical reaction, there are atoms …
The percentage atom economy of a reaction is calculated using this equation:.. . The percentage atom economy of a reaction is calculated using this equation:

Chemical reactions involve the conversion of reactants or raw materials into products... Total mass of all reactants = mass of desired product + mass of waste products. Equation (i) is identical to equation (ii) because by the law of mass conservation: Equation (i) is identical to equation (ii) because by the law of mass conservation:

At the very base of a chemical reaction, there are atoms … It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction.

The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … At the very base of a chemical reaction, there are atoms … Chemical reactions involve the conversion of reactants or raw materials into products. It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … The percentage atom economy of a reaction is calculated using this equation: Equation (i) is identical to equation (ii) because by the law of mass conservation: It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction.

It is an example of a green chemistry metric, which helps us understand the efficiency of a reaction. Table 4 experimental atom economy of equation 1: The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … The greater the value of the %atom economy, the less the amount of waste product produced. The equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Based on actual quantities of. Equation (i) is identical to equation (ii) because by the law of mass conservation: Total mass of all reactants = mass of desired product + mass of waste products. At the very base of a chemical reaction, there are atoms …. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

At the very base of a chemical reaction, there are atoms …. Based on actual quantities of. Chemical reactions involve the conversion of reactants or raw materials into products. Total mass of all reactants = mass of desired product + mass of waste products. Equation (i) is identical to equation (ii) because by the law of mass conservation: The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … Table 4 experimental atom economy of equation 1: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The percentage atom economy of a reaction is calculated using this equation: At the very base of a chemical reaction, there are atoms ….. Total mass of all reactants = mass of desired product + mass of waste products.

The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … Chemical reactions involve the conversion of reactants or raw materials into products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Table 4 experimental atom economy of equation 1: The percentage atom economy of a reaction is calculated using this equation: Total mass of all reactants = mass of desired product + mass of waste products. At the very base of a chemical reaction, there are atoms … The atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of … Equation (i) is identical to equation (ii) because by the law of mass conservation: Total mass of all reactants = mass of desired product + mass of waste products.

Table 4 experimental atom economy of equation 1: Based on actual quantities of. The percentage atom economy of a reaction is calculated using this equation:. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.
