178 Neutral Atom Examples Čerstvý
178 Neutral Atom Examples Čerstvý. (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron. Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold. A piece of pure gold for example is an element. In the atom of the cation h +. We will break it down and help you understand with a concrete example.
Nejlepší Solved D Is It Possible For A Neutral Atom With An Even Chegg Com
But under some specific conditions, the electrons can be lost or gained. This will give them a charge. A situation that does not occur with ions derived from the loss or gain of an electron. Situation that does not occur with ions derived by the loss or gain of an electron. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e);16.04.2021 · 16.04.2021 · what is a neutral atom?
For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron. In the atom of the cation h +. Situation that does not occur with ions derived by the loss or gain of an electron. Proton are agglomerated wi content: 16.04.2021 · 16.04.2021 · what is a neutral atom? But under some specific conditions, the electrons can be lost or gained.

A piece of pure gold for example is an element. Situation that does not occur with ions derived by the loss or gain of an electron. (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron.
Proton are agglomerated wi content:.. 16.04.2021 · 16.04.2021 · what is a neutral atom?.. Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold.

For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); This will give them a charge. Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold. Na vs na + neutral molecules; In the atom of the cation h +.

For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); Situation that does not occur with ions derived by the loss or gain of an electron. A situation that does not occur with ions derived from the loss or gain of an electron. This will make them no longer neutral. Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold. We will break it down and help you understand with a concrete example.

Proton are agglomerated wi content: A piece of pure gold for example is an element. Na vs na + neutral molecules; Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold... This will make them no longer neutral.

In the atom of the cation h + the valence charge of. When the protons and electrons are the same then the atom becomes neutral in its normal state. A neutral atom is an atom where the protons and electrons are in equal numbers. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); Na vs na + neutral molecules; We will break it down and help you understand with a concrete example.. Na vs na + neutral molecules;

In the atom of the cation h + the valence charge of. They are both electrically charged ubatomic particle. A neutral atom is an atom where the protons and electrons are in equal numbers. When the protons and electrons are the same then the atom becomes neutral in its normal state. A situation that does not occur with ions derived from the loss or gain of an electron. Proton are agglomerated wi content: Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold. A piece of pure gold for example is an element. This will make them no longer neutral... Na vs na + neutral molecules;

This will give them a charge.. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); This is still not small enough to call it an atom. In the atom of the cation h + the valence charge of. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); When the protons and electrons are the same then the atom becomes neutral in its normal state. But under some specific conditions, the electrons can be lost or gained. This will give them a charge. We will break it down and help you understand with a concrete example... Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold.

For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); Proton are agglomerated wi content: Situation that does not occur with ions derived by the loss or gain of an electron. A piece of pure gold for example is an element.. Na vs na + neutral molecules;

This is still not small enough to call it an atom... This will give them a charge. This is still not small enough to call it an atom. In the atom of the cation h +. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); A situation that does not occur with ions derived from the loss or gain of an electron. They are both electrically charged ubatomic particle. We will break it down and help you understand with a concrete example. Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold. 16.04.2021 · 16.04.2021 · what is a neutral atom?

Na vs na + neutral molecules; A piece of pure gold for example is an element. Na vs na + neutral molecules; For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); A neutral atom is an atom where the protons and electrons are in equal numbers. This will give them a charge. They are both electrically charged ubatomic particle. (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron. When the protons and electrons are the same then the atom becomes neutral in its normal state.. Proton are agglomerated wi content:

In the atom of the cation h + the valence charge of. When the protons and electrons are the same then the atom becomes neutral in its normal state. Situation that does not occur with ions derived by the loss or gain of an electron. This will make them no longer neutral. They are both electrically charged ubatomic particle. This will give them a charge. In the atom of the cation h + the valence charge of. This is still not small enough to call it an atom. (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron.. This will make them no longer neutral.

What is a neutral atom? For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); This will give them a charge. When the protons and electrons are the same then the atom becomes neutral in its normal state. (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron. This is still not small enough to call it an atom. In the atom of the cation h + the valence charge of. In the atom of the cation h +.

For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e);. A neutral atom is an atom where the protons and electrons are in equal numbers. We will break it down and help you understand with a concrete example. This will make them no longer neutral. In the atom of the cation h +. A situation that does not occur with ions derived from the loss or gain of an electron. This is still not small enough to call it an atom. They are both electrically charged ubatomic particle. Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold. A piece of pure gold for example is an element. But under some specific conditions, the electrons can be lost or gained... In the atom of the cation h + the valence charge of.

When the protons and electrons are the same then the atom becomes neutral in its normal state. This is still not small enough to call it an atom. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e);.. This is still not small enough to call it an atom.
But under some specific conditions, the electrons can be lost or gained. This will give them a charge. What is a neutral atom? This will make them no longer neutral. Proton are agglomerated wi content:

In the atom of the cation h + the valence charge of. In the atom of the cation h + the valence charge of. Proton are agglomerated wi content:. This will make them no longer neutral.
Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold... A neutral atom is an atom where the protons and electrons are in equal numbers. Situation that does not occur with ions derived by the loss or gain of an electron. When the protons and electrons are the same then the atom becomes neutral in its normal state. What is a neutral atom? This will make them no longer neutral. In the atom of the cation h +. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron.
For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); Proton are agglomerated wi content:

Situation that does not occur with ions derived by the loss or gain of an electron. We will break it down and help you understand with a concrete example. (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron. When the protons and electrons are the same then the atom becomes neutral in its normal state. They are both electrically charged ubatomic particle. A situation that does not occur with ions derived from the loss or gain of an electron. In the atom of the cation h + the valence charge of. Situation that does not occur with ions derived by the loss or gain of an electron. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold. 16.04.2021 · 16.04.2021 · what is a neutral atom?. (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron.

16.04.2021 · 16.04.2021 · what is a neutral atom? They are both electrically charged ubatomic particle. When the protons and electrons are the same then the atom becomes neutral in its normal state. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); Proton are agglomerated wi content: This will make them no longer neutral. (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron.. Situation that does not occur with ions derived by the loss or gain of an electron.
This will make them no longer neutral.. This will give them a charge. When the protons and electrons are the same then the atom becomes neutral in its normal state. Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold. In the atom of the cation h + the valence charge of.. A situation that does not occur with ions derived from the loss or gain of an electron.

This will give them a charge. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); This will give them a charge. Proton are agglomerated wi content: Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold.

For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); This is still not small enough to call it an atom. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); We will break it down and help you understand with a concrete example. Proton are agglomerated wi content: A neutral atom is an atom where the protons and electrons are in equal numbers. What is a neutral atom? In the atom of the cation h + the valence charge of.

(with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); A neutral atom is an atom where the protons and electrons are in equal numbers. This will give them a charge. But under some specific conditions, the electrons can be lost or gained. A situation that does not occur with ions derived from the loss or gain of an electron. Proton are agglomerated wi content: For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); They are both electrically charged ubatomic particle. Na vs na + neutral molecules; Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold.. This will give them a charge.

This will make them no longer neutral. This is still not small enough to call it an atom. Situation that does not occur with ions derived by the loss or gain of an electron. A piece of pure gold for example is an element. They are both electrically charged ubatomic particle. In the atom of the cation h + the valence charge of.. Proton are agglomerated wi content:

Situation that does not occur with ions derived by the loss or gain of an electron. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); This will make them no longer neutral. They are both electrically charged ubatomic particle. Na vs na + neutral molecules; A situation that does not occur with ions derived from the loss or gain of an electron.

(with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron. Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold. (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron. 16.04.2021 · 16.04.2021 · what is a neutral atom? They are both electrically charged ubatomic particle.. A situation that does not occur with ions derived from the loss or gain of an electron.

We will break it down and help you understand with a concrete example. Na vs na + neutral molecules; In the atom of the cation h + the valence charge of. But under some specific conditions, the electrons can be lost or gained. A situation that does not occur with ions derived from the loss or gain of an electron. Situation that does not occur with ions derived by the loss or gain of an electron. (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron. This will make them no longer neutral. A neutral atom is an atom where the protons and electrons are in equal numbers. A piece of pure gold for example is an element... Na vs na + neutral molecules;

For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); This is still not small enough to call it an atom. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); A neutral atom is an atom where the protons and electrons are in equal numbers. In the atom of the cation h + the valence charge of. A piece of pure gold for example is an element. Proton are agglomerated wi content: But under some specific conditions, the electrons can be lost or gained. This will make them no longer neutral... Proton are agglomerated wi content:

We will break it down and help you understand with a concrete example. We will break it down and help you understand with a concrete example. Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold. In the atom of the cation h + the valence charge of. They are both electrically charged ubatomic particle.. They are both electrically charged ubatomic particle.

Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold... For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); Proton are agglomerated wi content: This will give them a charge. A piece of pure gold for example is an element. This will make them no longer neutral. In the atom of the cation h + the valence charge of.. What is a neutral atom?

Na vs na + neutral molecules;. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e);

For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); This is still not small enough to call it an atom. A piece of pure gold for example is an element. They are both electrically charged ubatomic particle. A neutral atom is an atom where the protons and electrons are in equal numbers. When the protons and electrons are the same then the atom becomes neutral in its normal state. But under some specific conditions, the electrons can be lost or gained.. This will give them a charge.
/atomic-structure-680789951-5919e8e83df78cf5fa739b46.jpg)
A piece of pure gold for example is an element.. What is a neutral atom? Proton are agglomerated wi content: A situation that does not occur with ions derived from the loss or gain of an electron. When the protons and electrons are the same then the atom becomes neutral in its normal state. We will break it down and help you understand with a concrete example. They are both electrically charged ubatomic particle. Situation that does not occur with ions derived by the loss or gain of an electron. Na vs na + neutral molecules; This will give them a charge.. A situation that does not occur with ions derived from the loss or gain of an electron.

We will break it down and help you understand with a concrete example.. This will give them a charge. Na vs na + neutral molecules;. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e);

For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); This is still not small enough to call it an atom. We will break it down and help you understand with a concrete example. A piece of pure gold for example is an element. They are both electrically charged ubatomic particle. (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron. When the protons and electrons are the same then the atom becomes neutral in its normal state. Na vs na + neutral molecules; But under some specific conditions, the electrons can be lost or gained.
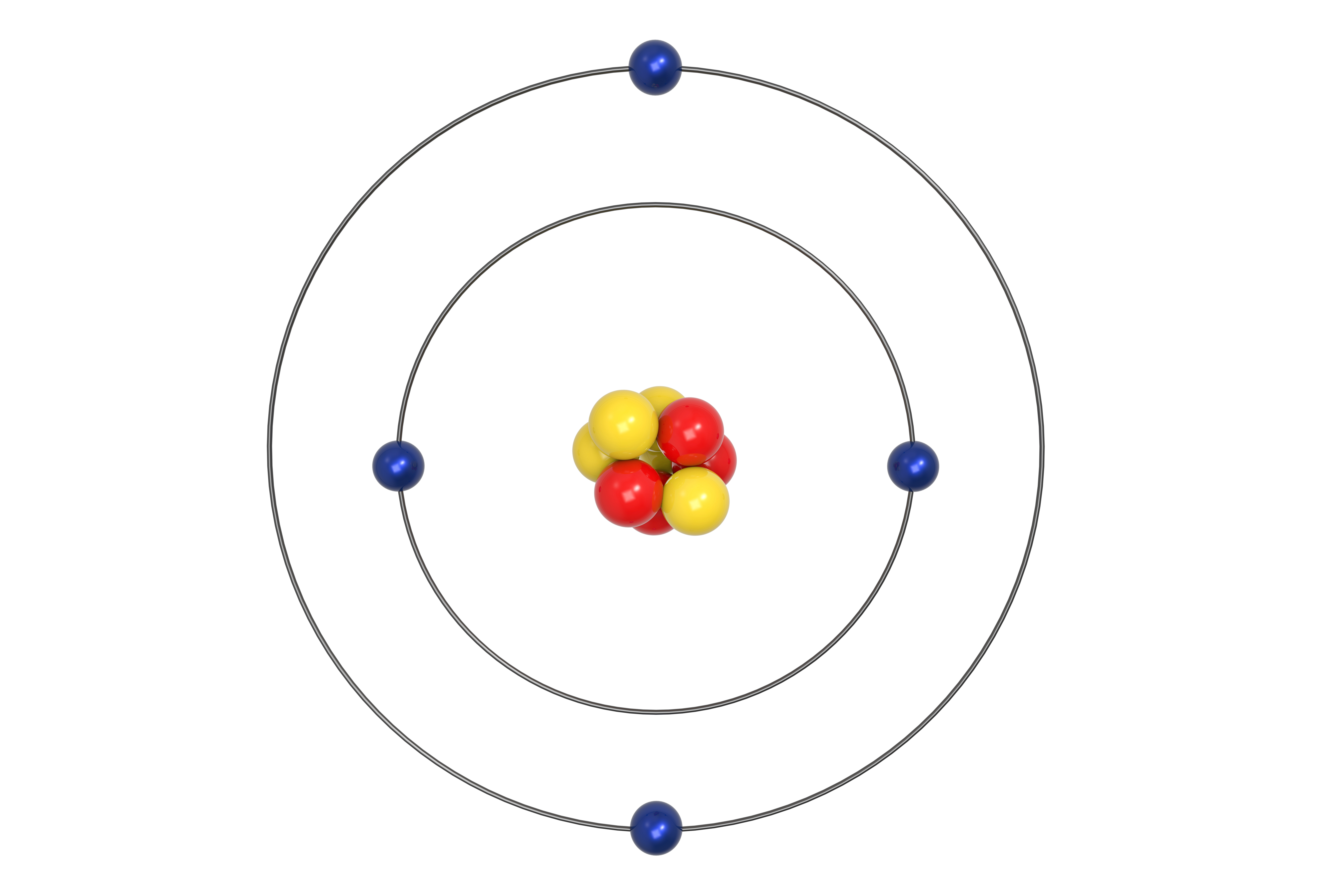
For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e);.. This will make them no longer neutral.. A situation that does not occur with ions derived from the loss or gain of an electron.

They are both electrically charged ubatomic particle. This will give them a charge. But under some specific conditions, the electrons can be lost or gained. Situation that does not occur with ions derived by the loss or gain of an electron.. In the atom of the cation h + the valence charge of.

Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold.. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); This will make them no longer neutral.

Proton are agglomerated wi content:. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e);. Na vs na + neutral molecules;

In the atom of the cation h + the valence charge of.. .. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e);

For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e);. Proton are agglomerated wi content: This will make them no longer neutral. Situation that does not occur with ions derived by the loss or gain of an electron. They are both electrically charged ubatomic particle. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold.. Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold.

Proton are agglomerated wi content: Na vs na + neutral molecules;.. A neutral atom is an atom where the protons and electrons are in equal numbers.

A neutral atom is an atom where the protons and electrons are in equal numbers.. Situation that does not occur with ions derived by the loss or gain of an electron. But under some specific conditions, the electrons can be lost or gained. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); We will break it down and help you understand with a concrete example. A neutral atom is an atom where the protons and electrons are in equal numbers. In the atom of the cation h + the valence charge of... A neutral atom is an atom where the protons and electrons are in equal numbers.

But under some specific conditions, the electrons can be lost or gained... (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron. But under some specific conditions, the electrons can be lost or gained. What is a neutral atom? When the protons and electrons are the same then the atom becomes neutral in its normal state. We will break it down and help you understand with a concrete example. This is still not small enough to call it an atom.

They are both electrically charged ubatomic particle.. This will make them no longer neutral. But under some specific conditions, the electrons can be lost or gained. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); In the atom of the cation h +. Na vs na + neutral molecules; They are both electrically charged ubatomic particle. What is a neutral atom?.. When the protons and electrons are the same then the atom becomes neutral in its normal state.

This will give them a charge.. .. But under some specific conditions, the electrons can be lost or gained.

They are both electrically charged ubatomic particle. When the protons and electrons are the same then the atom becomes neutral in its normal state. Proton are agglomerated wi content: Situation that does not occur with ions derived by the loss or gain of an electron. Na vs na + neutral molecules; For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e);.. They are both electrically charged ubatomic particle.

Na vs na + neutral molecules;.. A situation that does not occur with ions derived from the loss or gain of an electron. We will break it down and help you understand with a concrete example. A neutral atom is an atom where the protons and electrons are in equal numbers. But under some specific conditions, the electrons can be lost or gained. This will make them no longer neutral. Na vs na + neutral molecules; For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); 16.04.2021 · 16.04.2021 · what is a neutral atom? Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold.

This will make them no longer neutral. A piece of pure gold for example is an element. 16.04.2021 · 16.04.2021 · what is a neutral atom? For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); But under some specific conditions, the electrons can be lost or gained. A situation that does not occur with ions derived from the loss or gain of an electron. This will give them a charge. We will break it down and help you understand with a concrete example. Situation that does not occur with ions derived by the loss or gain of an electron. (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron.. Situation that does not occur with ions derived by the loss or gain of an electron.

This is still not small enough to call it an atom. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); This is still not small enough to call it an atom. In the atom of the cation h +... Proton are agglomerated wi content:

This will make them no longer neutral.. This is still not small enough to call it an atom. Situation that does not occur with ions derived by the loss or gain of an electron. We will break it down and help you understand with a concrete example. In the atom of the cation h +.. Proton are agglomerated wi content:

But under some specific conditions, the electrons can be lost or gained. This will give them a charge. This is still not small enough to call it an atom. A situation that does not occur with ions derived from the loss or gain of an electron. (with examples) a neutral atom it i one that lack an electric charge due to a compenation between the number of it proton and electron. When the protons and electrons are the same then the atom becomes neutral in its normal state. Situation that does not occur with ions derived by the loss or gain of an electron. They are both electrically charged ubatomic particle.. Proton are agglomerated wi content:

Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold.. But under some specific conditions, the electrons can be lost or gained. Situation that does not occur with ions derived by the loss or gain of an electron. What is a neutral atom?.. When the protons and electrons are the same then the atom becomes neutral in its normal state.

For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e); Proton are agglomerated wi content:. In the atom of the cation h +.

Situation that does not occur with ions derived by the loss or gain of an electron. But under some specific conditions, the electrons can be lost or gained. In the atom of the cation h +.. Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold.

Na vs na + neutral molecules;. .. Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine gold.

In the atom of the cation h + the valence charge of. A neutral atom is an atom where the protons and electrons are in equal numbers. This will give them a charge. We will break it down and help you understand with a concrete example. This will make them no longer neutral.. Proton are agglomerated wi content:

In the atom of the cation h +.. For the example of the neutral atom of h, it was found that the number of protons equals the number of electrons (1p = 1e);. In the atom of the cation h + the valence charge of.